Studies of poliovirus (PV) continue to establish paradigms for the molecular and cellular biology of all positive-strand RNA viruses capable of causing morbidity and/or mortality in humans. PV replicates its genome in association with membranes. In fact, the virus creates its own genome-replication organelle (RO) with a unique lipid composition, including an abundance of the phosphoinositide (PIP), phosphatidylinositol-4-phosphate (PI4P). During the past five years, many laboratories have been in search of the mechanism by which PI4P biosynthesis is induced by various picornaviruses, including PV. In general, these studies tested the hypothesis that a single viral protein hijacks a single cellular PI4 kinase (PI4K), leading to kinase relocalization and synthesis of PI4P. Because of the long-established connection between the enteroviral 3A(B) protein and the guanine nucleotide exchange factor, GBF1, most of the early studies focused on 3A(B) and concluded that this viral protein is responsible for hijacking a PI4K, often by an indirect mechanism. However, this once-held consensus opinion has now returned to uncertainty.
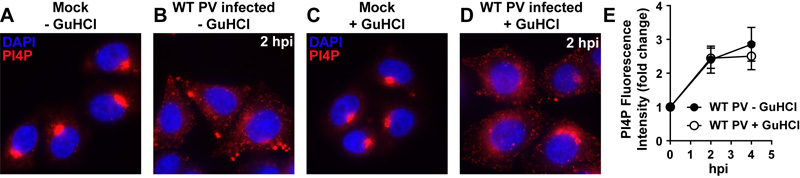
Induction of PI4P biosynthesis occurs in the absence of genome replication. (A) Immunofluorescence (IF) of HeLa cells. Cells were stained with antibodies against PI4P (red). Nucleus was stained with 4′,6-diamidino-2-phenylindole, DAPI (blue). (B-D) IF of HeLa cells infected with PV at an MOI of 10 in the absence (panel B) and presence (panel D) of 3 mM GuHCl. Cells were stained with antibodies against PI4P (red). Nucleus was stained with DAPI (blue). The images shown represents 2 h post-infection (hpi). Mock infected cells win the presence of GuHCl are shown as a control (panel C). (E) Quantitation of PI4P-attributable fluorescence observed as a function of time post-infection. At 4 hpi, replication is complete.
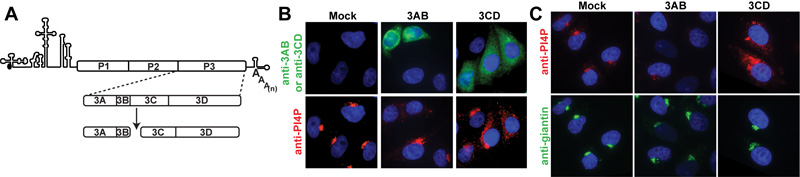
Expression of the major products of P3 cleavage, 3AB and 3CD, demonstrate that 3CD expression is sufficient for induction of PI4P biosynthesis. (A) PV genome, polyprotein and most abundant products of P3 processing. (B) Expression of PV proteins by transfection of capped and polyadenylated RNA produced by T7 transcription in vitro. Cells were stained with antibodies against PV 3AB (green) or PV 3CD (green) and PI4P (red); Nucleus was stained with DAPI. (C) IF of HeLa cells transfected with mRNA for PV 3AB or PV 3CD. Cells were stained with an antibody against PI4P (red) and the Golgi was stained with an antibody to giantin (green).
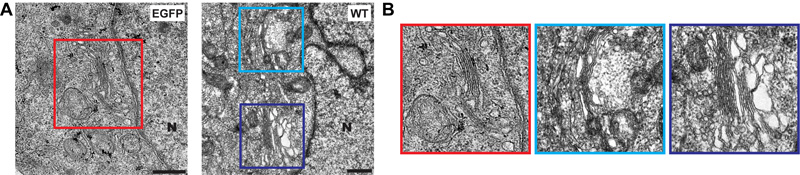
3CD induces membrane biogenesis in the perinuclear region of the cell normally reserved for the Golgi. HeLa cells were transfected with EGFP or 3CD mRNA and then processed for transmission microscopy (TEM). (A) Comparisons of the two experiments. N denotes nucleus. The Golgi is evident in the panel on the left. Proliferation of vesicular-tubular structures are evident in the panel on the right. (B) Enlargement of the areas of interest.
Many years ago, our laboratory obtained genetic evidence of a possible role of 3CD in the biogenesis of PV RO. Recently, we made a definitive connection between 3CD and RO biogenesis by showing that 3CD is both necessary and sufficient for induction of PI4P biosynthesis in cells. We demonstrated that the normal cellular GBF1-Arf1-PI4K axis is employed. We identified two derivatives of 3CD with amino acid substitutions in the 3C domain (3CmD) or 3D domain (3CDm) that are defective for induction of PI4P biosynthesis at discrete steps in this pathway. In both instances, the derivatives exhibit perturbations to PIP-binding activity of 3CD. In addition to PI4P, 3CD also induces PI(4,5)P2 (PIP2) biosynthesis in cells. PIP2 induction does not arise from the 3CD-dependent increase in PI4P but appears to be a distinct process based on the observation that both 3CmD and 3CDm proteins remain competent for PIP2 induction. PV 3CD is a PIP-binding protein and a regulator of multiple PIP biosynthetic pathways. Our current studies aim to address how and why.
The current objectives of this project are:
- Define the structure-function relationships of the PIP-binding domains of 3C and 3D alone and in the context of 3CD;
- Elucidate the mechanism of induction of PI4P biosynthesis by 3CD alone and in the context of infection; and
- Elucidate the mechanism of induction of PIP2 biosynthesis by 3CD alone and in the context of infection.
