The enteroviral genome encodes a polyprotein, which is divided into three functional regions: P1, P2, and P3. The P2 region is comprised of three protein domains: 2A, 2B, and 2C. For decades, 2C protein has been a validated target for anti-enteroviral therapeutics. Guanidine inhibits PV genome replication, and resistance maps to 2C-coding sequence. A hydantoin-containing compound inhibits infection by PV at a stage of the lifecycle after genome replication, perhaps encapsidation, and resistance again maps to 2C-coding sequence. More recently, screens to discover anti-enteroviral compounds have identified even more compounds targeting 2C protein, based on the genetics of resistance. However, essentially nothing is known about the biochemical/biophysical mechanism(s) of action of these compounds []. The dearth of mechanistic studies reflects the absence of robust systems to study 2C protein in vitro.
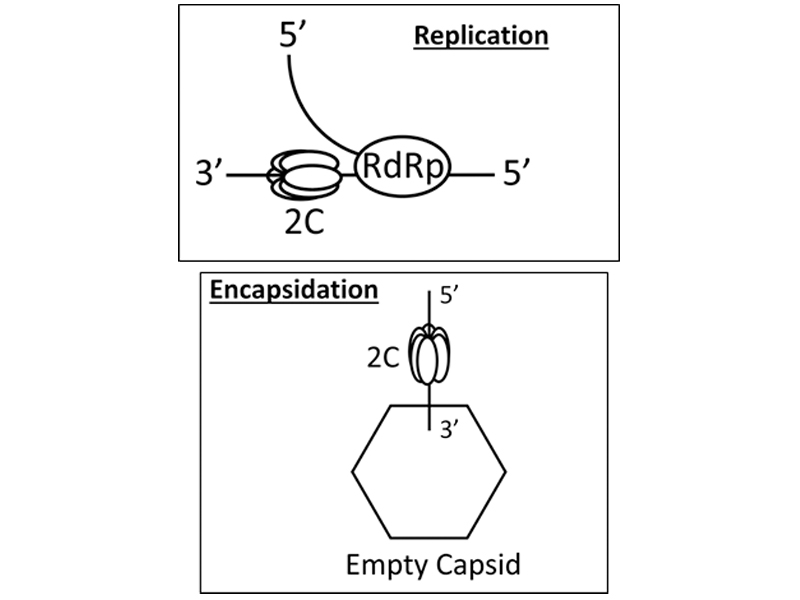
Biochemical studies of the 2C protein have been complicated by the presence of a long amphipathic alpha helix on its amino terminus, which has complicated expression and purification. Our laboratory has succeeded at expressing and purifying soluble, full-length PV 2C protein to at least 95% purity. Solubility can be maintained using dialyzable detergents at concentrations below the critical micelle concentration, thus enabling biochemical and biophysical analyses. The protein exhibits ATPase activity that is stimulated more than 25-fold by the presence of single-stranded RNA but not by the presence of ssDNA. Guanidine inhibits ATPase activity in the absence and presence of RNA in a manner predicted by cell-based studies; hydantoin does not. These studies suggest distinct mechanisms of action for these compounds.
With these accomplishments, the new structural data for a truncated derivative of 2C protein, and provocative conformations of 2C protein derived from computational modeling and molecular dynamics simulations, we are now poised to provide unique insight into the biochemistry, biophysics, and biology of the enterovirus 2C protein essential to target this protein with inhibitors that exhibit pan-enteroviral activity.
